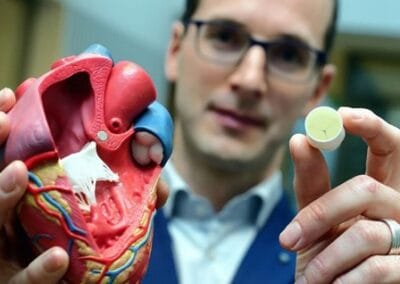
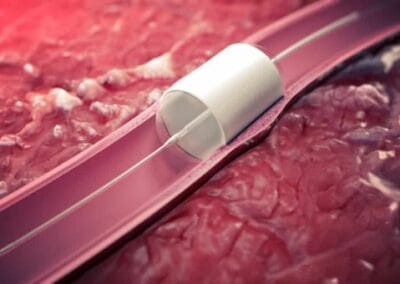
Brabant is at the heart of various developments in the field of biomaterials and regenerative medicine. Artificial heart valves that are naturally absorbed by the body, arteries that repair themselves without invasive surgery, jawbone that can grow with a gel and all kinds of other self-healing materials: the world of biomaterials and regenerative medicine is full of promise. And Brabant is at the heart of various developments in this area. Companies such as Xeltis, Vivolta, Stentit, Osteo Pharma and FujiFilm are now making their mark on this new sector of medical technology.
Two developments are currently giving an extra boost to this trend. In Brabant itself SBMC is being formed with the Brabant Development Company (BOM), Eindhoven University of Technology, the Province of Noord-Brabant, Pivot Park Oss, Brainport Industries Campus and dozens of companies as participants.
In addition, there is a collaboration at national level between Leiden, Utrecht, Eindhoven and Maastricht for the realization of various development and production lines in regenerative medicine. This national network is part of ‘RegMed XB’: regenerative medicine crossing borders, an international initiative in regenerative medicine (RG) that connects top clusters in the Netherlands and Flanders.
Within RegMed XB, researchers, companies and governments in the Netherlands and Flanders are joining forces to create a new industry here, including new innovative companies based on knowledge-intensive labour. RegMed XB is ambitious: it wants to create a nationwide RG pilot factory, as the basis for a new manufacturing industry that makes RG therapies, production technology and production equipment for the whole world. A subsidy of €56 million from the Growth Fund (on a total investment of more than 200 million until 2027) should give RegMed XB an acceleration. This brings the combined pilot factory with thematic focus on stem cells (Leiden), microtissue (Utrecht), biomaterials (Eindhoven) and complete organs (Maastricht) a lot closer.
Benefit too 
The development of the Brabant biomaterials center is gaining momentum by carrying out part of the Growth Fund project for the RG pilot plant, but quartermaster Jan Rietsema emphasizes that SBMC would have started anyway. “Our Center focuses on hydrogels and biodegradable implants. Biomaterials play a major role in the production and deployment of regenerative therapies. Hydrogels are used to accelerate cell culture and to deliver drugs or cells and tissues into the body. The latter also applies to biodegradable implants. These are biomaterials specifically constructed to support the growth of new functional tissue in the body. They dissolve in the body so they don’t have to be surgically removed.”
SBMC wants to make the move towards industrialization, partly thanks to the impulse of the Growth Fund. Rietsema: “A lot is already possible at a scientific level, but success on a petri dish is not yet a successful company.” And that’s what it should lead to. Unlike in Leiden, Utrecht and Maastricht, the role of the business community in the Brabant leg of the collaboration is therefore relatively strong. “About forty Brabant companies are already involved in the Center . We have only just been established and are still in a very dynamic phase. Although we are very industry-driven, the role of TU Eindhoven is also essential. And with the input of the province, the triple helix is complete.”
Apart from setting up a foundation and putting together a board, the focus is mainly on the content. The first ideas have already been collected for this: there were workshops with the forty companies and now it comes down to further detailing of the plans – because now they only exist in outline. “The goal is a joint development facility where companies can develop applications together. There are already many existing collaborations. For example, there are close ties between Vivolta with Stentit and Xeltis. We want this to take place as much as possible in open innovation, whereby we will have to pay attention to protection of IP and patents. We do have experience with that, so that will probably be fine, but it does require attention. Also from the companies themselves.”
High demands
High demands are made on both the materials and the method of production – such as the electrospinning of fibers and 3D bioprinting. Rietsema: “Everything must be clinically validated and produced under strictly controlled conditions of Good Manufacturing Practice.”
SBMC plans to realize two facilities:
A development facility, whose central location will preferably be near Eindhoven University of Technology. Here, parties work together on optimized production and assembly of biomaterials (including hydrogels), on functional testing, assessment and prediction of implant function, upscaling problems and the restrictions for clinical production.
A pilot production facility elsewhere, where parties collaborate on the industrial production of biomaterials and biodegradable implants.
The revenue model for this part of the RG pilot factory is a combination of:
Sales (mainly of hydrogels)
Contract manufacturing (of biodegradable implants)
Consultancy/support in the development of products and production technology
Customers are companies that want to purchase biomaterials, organizations developing new production technology, and other players in the RG pilot plant using the biomaterials in their pilot lines. Through this model, a manufacturing industry around biomaterials can develop — with the Brabant production cluster for hydrogels and biodegradable implants as the focal point. The province of Noord-Brabant and TU Eindhoven have taken the lead in realizing the infrastructure, with SBMC as the basis for a production ecosystem aiming for a leading position in Europe.
Pioneers
Eindhoven University of Technology is named among the Dutch pioneers in regenerative medicine and internationally progressive in the field of biomaterials for RG applications. Additionally, there is a strong financial basis: BOM has experience with large funds in biotechnology and works closely with TU/e and other early-phase investors such as Braventure and the Brabant Startup Fund.
Beyond TU/e, other important players in building a high-tech manufacturing industry around biomaterials are Pivot Park Oss and Brainport Industries Campus (BIC) in Eindhoven. Pivot Park Oss is an initiative of a major pharmaceutical company in collaboration with the province, municipality, and ministries; BIC is a campus where companies share knowledge of product development and technology, working closely together in research and development. The BOM played a major role in the creation of the innovation program at BIC.
Of the approximately forty companies already associated with SBMC, several stand out:
Xeltis: Builds on TU/e technology to develop implantable, growing heart valves. The artificial valve is eventually absorbed, and the body builds a natural, functional valve around it. Xeltis’ bioabsorbable polymers are also used for the formation of blood vessels. The valves are in clinical trials in the EU, Asia and the US, and the first patients have now reached their 5-year follow-up. Xeltis has raised nearly US$100 million in several funding rounds.
Stentit: A spin-off of TU/e focused on regenerating arteries without invasive surgery, via a regenerative stent. The company is advancing toward clinical trials.
Vivolta (formerly IME Medical Electrospinning): Offers medical solutions enabling the human body to regenerate itself. Implants based on nanofibres support tissue and organ repair. Vivolta has evolved into a MedTech company developing medical devices, tissue engineering products and drug-delivery solutions. Its technologies are already used by firms like Xeltis and Stentit.
Osteo Pharma: Develops products from Pivot Park Oss for orthopedic and dental applications — for example bone-fracture healing and bone-defect repair. Their OsteoActivator technology delivers bone-healing compounds via a biodegradable carrier, promoting bone growth and reducing breakdown. Their dental variant is ready for clinical trial; preclinical studies are ongoing for other applications.
Fujifilm: Based in Tilburg, this major international company is active in biomedical materials for regenerative medicine. At the end of 2021, they opened a new Life Science Manufacturing factory to produce cell culture media — essential for bioproduction and cell & gene therapies. The factory strengthens supply chains in Europe, reduces transport (greener logistics) and supports production of medically relevant materials locally.